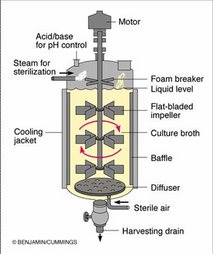
Introduction
Pharmaceutical proteins produced via fermentation in transgenic microbes or mammalian cell culture systems provide economical systems for production of therapeutic proteins. These include antibodies, vaccines, blood proteins, etc.
Biopharmaceuticals are medical drugs (see pharmacology) produced using biotechnology. They are proteins (including antibodies), nucleic acids (DNA, RNA or antisense oligonucleotides) used for therapeutic or in vivo diagnostic purposes, and are produced by means other than direct extraction from a native (non-engineered) biological source
Dozens of new pharmaceuticals produced via fermentation in transgenic microbes have been approved for therapeutic use in the USA. Hundreds of additional biotech drug candidates are in various stages of research or clinical trials. Fermentation systems can be scaled up to produce quantities of pharmaceuticals that are difficult or impossible to produce via traditional methods. Pharmaceutical quality may also be improved. For example, pharmaceuticals produced from blood must be carefully purified to ensure no transmission of viruses as accidental contaminants in the pharmaceutical product. Microbial systems that do not allow human viruses to replicate enable pharmaceutical production with little or no risk of virus contamination.
The total biotech exports in 2005-06 were at Rs 3,357.17 crore, while the domestic business reported Rs 3,163.83 crore in sales. The exports accounted for 51.48 percent share of the total industry. BioPharma exports accounted for 74.33 percent of the total exports of Rs 3,357.17 crore, clocking Rs 2,495.24 crore in revenues. BioAgri's share of exports was the lowest at 1.07 percent and exports from this segment stood at Rs 35.88 crore. BioServices overseas revenues were Rs 684 crore. BioIndustrial and Bioinformatics sector accounted for 1.23 percent and 3 percent share of the total exports respectively.
Classification of biopharmaceuticals
Definition of Fermentation:
Fermentation technology is the oldest of all biotechnological processes. The term is derived from the Latin verb fevere, to boil--the appearance of fruit extracts or malted grain acted upon by yeast, during the production of alcohol.
Fermentation is a process of chemical change caused by organisms or their products, usually producing effervescence and heat.
Microbiologists consider fermentation as 'any process for the production of a product by means of mass culture of micro-organisms'.
Biochemists consider fermentation as 'an energy-generating process in which organic compounds act both as electron donors and acceptors'; hence fermentation is ‘an anaerobic process where energy is produced without the participation of oxygen or other inorganic electron acceptors’.
One process by which carbon-containing compounds are broken down in an energy yielding process. Fermentation occurs during times of low oxygen supply and is therefore known as a type of anaerobic respiration.
The anaerobic enzymatic conversion of organic compounds, especially carbohydrates, to simplercompounds , especially to ethyl alcohol, resulting in energy in the form of adenosine Triphosphate (ATP). The process is used in the production of alcohol, bread, vinegar and other food or industrial products. It differs from respiration in that organic substances rather than molecular oxygen are used as electron acceptors
Fermentation occurs widely in bacteria and yeasts, the process usually being identified by the product formed, for example, acetic, alcoholic, butyric and lactic fermentation are those that result in the formation of acetic acid, alcohol, butyric acid and lactic acid, respectively.
Commercially important Fermentation - Microbial cells or Biomass as the product: Eg. Bakers Yeast, Lactic acid bacillus, Bacillus sp.
- Microbial Enzymes: Catalase, Amylase, Protease, Pectinase, Glucose isomerase, Cellulase, Hemicellulase, Lipase, Lactase, Streptokinase etc.
- Microbial metabolites :
- Primary metabolites – Ethanol, Citric acid, Glutamic acid, Lysine, Vitamins, Polysaccharides etc.
- Secondary metabolites: All antibiotic fermentation
- Recombinant products : Insulin, HBV, Interferon, GCSF, Streptokinase
- Biotransformations: Eg. Phenyl acetyl carbinol,Steroid Biotransformation
Nutrient sources for industrial fermentation
Medium for Industrial Fermentations
Any Microbe requires Water, Oxygen, Energy source, Carbon source, Nitrogen source and Micronutrients for the growth.
Carbon & Energy source + Nitrogen source + O2 + other requirements → Biomass + Product + byproducts + CO2 + H2O + heat
Nutrient sources for industrial fermentation
Carbon source
Glucose Corn sugar, Starch, Cellulose
Sucrose Sugarcane, Sugar beet molasses
Lactose Milk whey
Fats Vegetable oils
Hydrocarbons Petroleum fractions
Nitrogen source
Protein Soybean meal, Cornsteep liquor, Distillers' solubles
Ammonia Pure ammonia or ammonium salts
Nitrate Nitrate salts
Nitrogen Air
Phosphorous source Phosphate salts
Trace elements : Fe, Zn, Cu, Mn, Mo, Co
Antifoaming agents : Esters, Fatty acids, Silicones, Sulphonates, Polypropylene
Buffers: Calcium carbonate, Phosphates
Growth factors: Some microorganisms cannot synthesize the required cell components themselves and need to be supplemented: E.g. Thiamine, Biotin, Calcium pentothenate
Precursors: Directly incorporated into the desired product: Phenyl ethylamine into Benzyl penicillin, Phenyl acetic acid into Penicillin G
Inhibitors: To get the specific products: e.g. Sodium barbital for Rifamycin
Inducers: Majority of the enzymes are inducible and are synthesized in response of inducers: e.g. Starch for Amylases, Maltose for Pollulanase, Pectin for Pectinase
Chelators: Chelaters are the chemicals used to avoid the precipitation of metal ions. Chelaters like EDTA, Citric acid, Polyphosphates are used in low concentrations.
For more details on industrial fermentation read
1. Biochemical Engineering Fundamentals by J.E. Bailey and P.F. Ollis, McGraw Hill Publication 2. . Principles of fermentation technology by Stansbury, P.F., A. Whitaker and S.J. Hall, 1997
Biotechnology Companies Developing Pharmaceuticals via Fermentation in Transgenic Microbes or Mammalian Cell Cultures
PRODUCTS OF FERMENTATION PROCESSES
The growth of micro-organisms or other cells results in a wide range of products. Each culture operation has one or few set objectives. The process has to be monitored carefully and continuously, to maintain the precise conditions needed and recover optimum levels of products. Accordingly, fermentation processes aim at one or more of the following:
a) production of cells (biomass) such as yeasts;
b) extraction of metabolic products such amino acids, proteins (including enzymes), vitamins, alcohol, etc., for human and/or animal consumption or industrial use such as fertiliser production;
c) modification of compounds (through the mediation of elicitors or through biotransformation); and
d) production of recombinant products.
A. MICROBIAL BIOMASS
Microbial biomass is produced commercially as single cell protein (SCP) using such unicellular algae as species of Chlorella or Spirulina for human or animal consumption, or viable yeast cells needed for the baking industry, which was also used as human feed at one time. Bacterial biomass is used as animal feed. The biomass of Fusarium graminearum is also produced, for a similar use.
B. MICROBIAL METABOLITES
i) Primary metabolites:
During the log or exponential phase organisms produce a variety of substances that are essential for their growth, such as nucleotides, nucleic acids, amino acids, proteins, carbohydrates, lipids, etc., or by- products of energy yielding metabolism such as ethanol, acetone, butanol, etc. This phase is described as the tropophase, and the products are usually called primary metabolites. Commercial examples of such products are given in Table 2.
TABLE 2
Examples of commercially produced primary metabolites
Primary Organism Significance
Metabolite
Ethanol Saccharomyces cerevisiae alcoholic beverages
Kluyveromyces fragilis
Citric acid Aspergillus niger food industry
Acetone and Clostridium
butanol acetobutyricum solvents
Lysine Corynebacterium nutritional additive
Glutamic acid glutamacium flavour enhancer
Riboflavin Ashbya gossipii nutritional
Eremothecium ashbyi
Vitamin B12 Pseudomonas denitrificans nutritional
Propionibacterium shermanii
Dextran Leuconostoc mesenteroides industrial
Xanthan gum Xanthomonas campestris industrial
ii) Secondary metabolites:
Organisms produce a number of products, other than the primary metabolites. The phase, during which products that have no obvious role in metabolism of the culture organisms are produced, is called the idiophase, and the products are called secondary metabolites.
In reality, the distinction between the primary and secondary metabolites is not a straightjacket situation. Many secondary metabolites are produced from intermediates and end products of secondary metabolism. Some like those of the Enterobacteriaceae do not undergo secondary metabolism. Examples of secondary metabolites are given in Table 3.
TABLE 3
Examples of commercially produced secondary metabolites
Metabolite Species Significance
Penicillin Penicillium chrysogenum antibiotic
Erythromycin Streptomyces erythreus antibiotic
Streptomycin Streptomyces griseus antibiotic
Cephalosporin Cephalosporium acrimonium antibiotic
Griseofulvin Penicillium griseofulvin antifungal antibiotic
Cyclosporin A Tolypocladium inflatum immunosuppressant
Gibberellin Gibberella fujikuroi plant growth regulator
Secondary metabolism may be repressed in certain cases. Glucose represses the production of actinomycin, penicillin, neomycin and streptomycin; phosphate represses streptomycin and tetracyclin production. Hence, the culture medium for secondary metabolite production should be carefully chosen.
C. PRODUCTION ENZYMES
Industrial production of enzymes is needed for the commercial production of food and beverages. Enzymes are also used in clinical or industrial analysis and now they are even added to washing powders (cellulase, protease, lipase). Enzymes may be produced by microbial, plant or animal cultures. Even plant and animal enzymes can be produced by microbial fermentation. While most enzymes are produced in the tropophase, some like the amylases (by Bacillus stearothermophilus) are produced in the idiophase, and hence are secondary metabolites. Examples of enzymes produced through fermentation processes are given in Table 4.
TABLE 4
Examples of commercially produced enzymes
Organism Enzyme
Aspergillus oryzae Amylases
Aspergillus niger Glucamylase
Trichoderma reesii Cellulase
Saccharomyces cerevisiea Invertase
Kluyveromyces fragilis Lactase
Saccharomycopsis lipolytica Lipase
Aspergillus species Pectinases and proteases
Bacillus species Proteases
Mucor pusillus Microbial rennet
Mucor meihei Microbial rennet
D. FOOD INDUSTRY PRODUCTS
A very wide range of innumerable products of the food industry, such as sour cream, yoghurt, cheeses, fermented meats, bread and other bakery products, alcoholic beverages, vinegar, fermented vegetables and pickles, etc., are produced through microbial fermentation processes. The efficiency of the strains of the organisms used, and the processes are being continuously improved to market quality products at more reasonable costs.
E. RECOMBINANT PRODUCTS
Recombinant DNA technology has made it possible to introduce genes from any organism into micro-organisms and vice versa, resulting in transgenic organisms and the latter are made to produce the gene product. Genetically manipulated Escherichia coli, Saccharomyces cerevisiae, other yeasts and even filamentous fungi are now being used to produce interferon, insulin, human serum albumin, and several other products.
F. BIOTRANSFORMATION
Production of a structurally similar compound from a particular one, during the fermentation process is transformation, or biotransformation, or bioconversion. The oldest instance of this process is the production of acetic acid from ethanol.
Immobilised plant cells may be used for biotransformation. Using alginate as the immobilising polymer, digitoxin from Digitalis lanata was converted into digoxin, which is a therapeutic agent in great demand. Similarly, codeinone was converted into codeine and tyrosine from Mucuna pruriens was converted into DOPA.
G. ELICITORS
It is possible to induce production or enhance production of a compound in cultures by using elicitors, which may be micro-organisms. For example, Saccharomyces cerevisiae was an efficient elicitor in the production of glyceollin (Glycine max) and berberine (Thalictrum rugosum). Rhizopus arrhizus trebled diosgenin production by Dioscorea deltoidea. The production of morphine and codeine by Papaver somniferum was increased 18 times by Verticillium dahliae.
EXAMPLES :
Vitamins
- Figure 11.13, Vitamin B12
- Used as supplements for human food and animal feeds
- Nearly $1B/year
- Synthesized chemically, but some by biocatalysis
- Selected high-yield strains for B12 produce up to 60 mg/L
- For riboflavin, up to 7 g/L
Amino Acids in the Food Industry (a short list)
- L-Glutamate (MSG) flavor enhancer
- Aspartame (phe + asp) sweetener
- L-Lysine nutritive additive
- DL- Methionine nutritive additive
Industrial Production of Lysine
- Figure 11.14a, Biochemical pathway of aspartate to lysine
- Figure 11.14g, Structures of lysine and S-aminoethylcysteine
- Overproducing strain: Brevibacterium flavum can produce over 60 g lysine/liter
Bioconversion
- Supplement to organic synthesis
- Growth in a large fermentor
- Chemical to be converted added at appropriate time
- Incubation
- Conversion
- Extraction
- Purification of product
- Figure 11.15, Cortisone production using a microorganism
Enzymes and their Markets
- Pharmaceuticals
- Agricultural
- Genetically engineered crops, animal feed ($5.9B)
- Chemical
- Fine chemicals ($45B), specialty chemicals, and polymers
- Industrial
- Consumer products, oil well breakers
Enzymes and Industrial Uses
- Amylases = used in bread, glucose manufacture, in detergents
- Proteases = used in bread, stain removal, meat tenderizing, contact cleaning solns
- Cellulases = used in fabric softening, “stone-washed” jeans, detergents
- Lipases = used in detergents, break-down of fats
- DNA polymerases = used in biotechnology (PCR)
“Extremo”zymes: Enzymes that function under harsh industrial and environmental conditions
- There is a need for enyzmes that catalyze desired (and often very specific) chemical conversions under typically harsh industrial and environmental conditions:
- Low- and High pH
- Cold to very hot temperatures
- Activity in non-aqueous solutions
Thermostable Enzymes
- Figure 11.16b, Thermostability of pullulanase from Pyrococcus woesei
- Examples of “Extremo”zymes
- Thermus aquaticus
- DNA polymerase @ 75 – 95 C
- Thermus thermophilus
- Thermococcus litoralis
- Pyrococcus furiosus
- Psychrophiles – cold active enzymes
- Acidophiles/Alkaliphiles – low-/high pH
- Halophiles – High salt concentrations